Methodology Studies
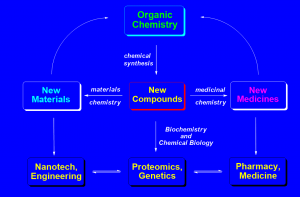
- There is an ever-growing need for novel methodologies for the efficient construction of useful compounds. The Pericyclic reactions are the most reliable and operationally simple. We are currently involved in the development of tandem sequences and novel variants for application in the synthesis of complex natural and unnatural compounds.
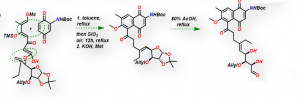
Cycloaddition-3,3 sigmatropic rearrangements for assembly of naphthoquinones
- We are involved in the design and development of novel variants of the aza-Nazarov reaction. For example, the quinazolinonyl enones are siphoned into two types of pyrroloquinazolinones i.e., linear vs angular, through the intrinsic divergence built into the enones and through the identification of diverse reaction conditions. This methodology would find application in the diastereoselective synthesis of C-ring substituted vasicinones and C-ring substituted luotonins.

Novel Aza-Nazarov Cyclization of Quinazolinonyl Enones
- We have also been engaged in the development of variously substituted Imiazopyridines, Imiazopyrimidines, and Imiazopyridazines. We have successfully achieved the synthesis of 4(5)- substituted -2-amino imidazoles employing imidazo [1,2-a]pyrimidines as the surrogate for 2-amino-1H-imidazoles.
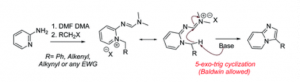
Facile synthesis of 3-substituted imidazo[1,2-a]pyridines

Facile synthesis of 2-vinyl imidazo[1,2-a]pyrimidines
